About the entry
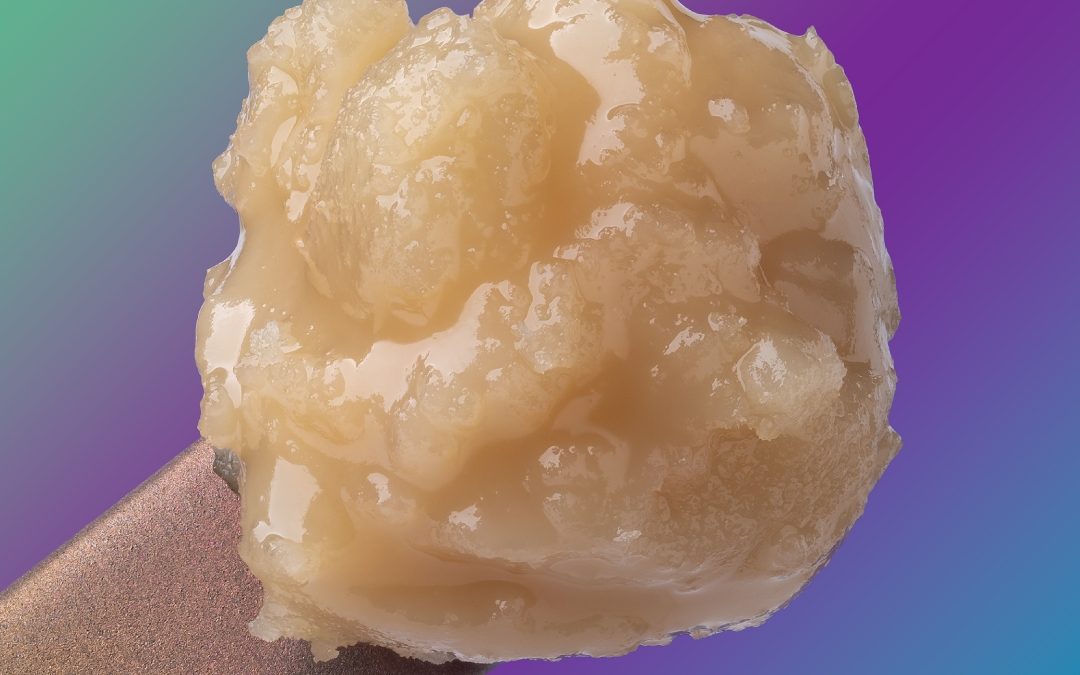
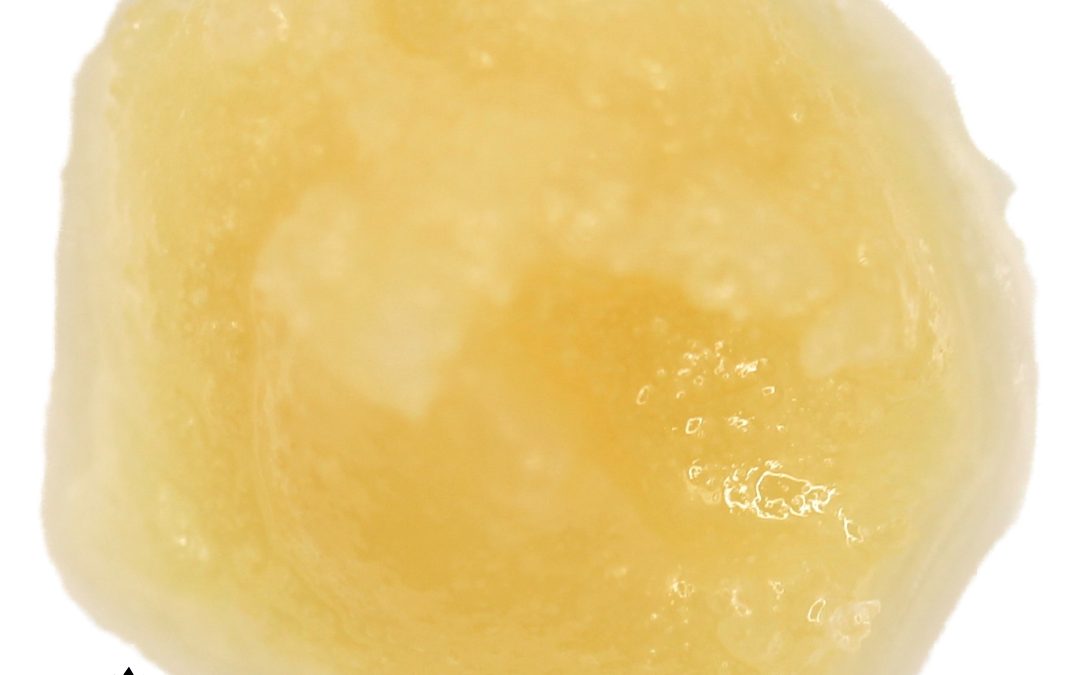
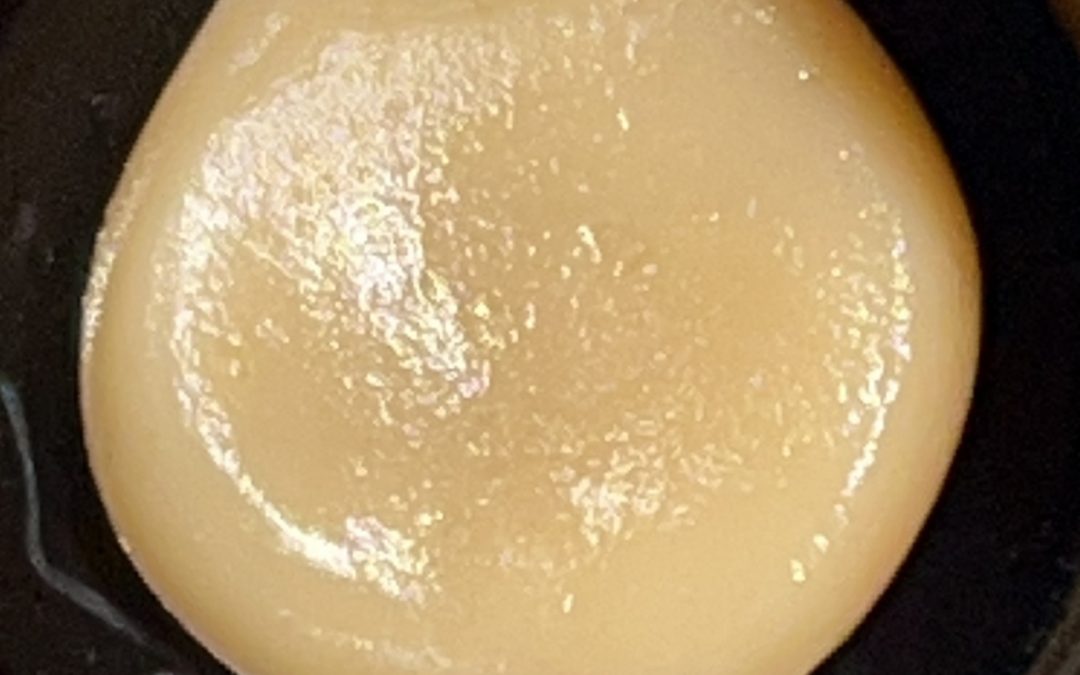
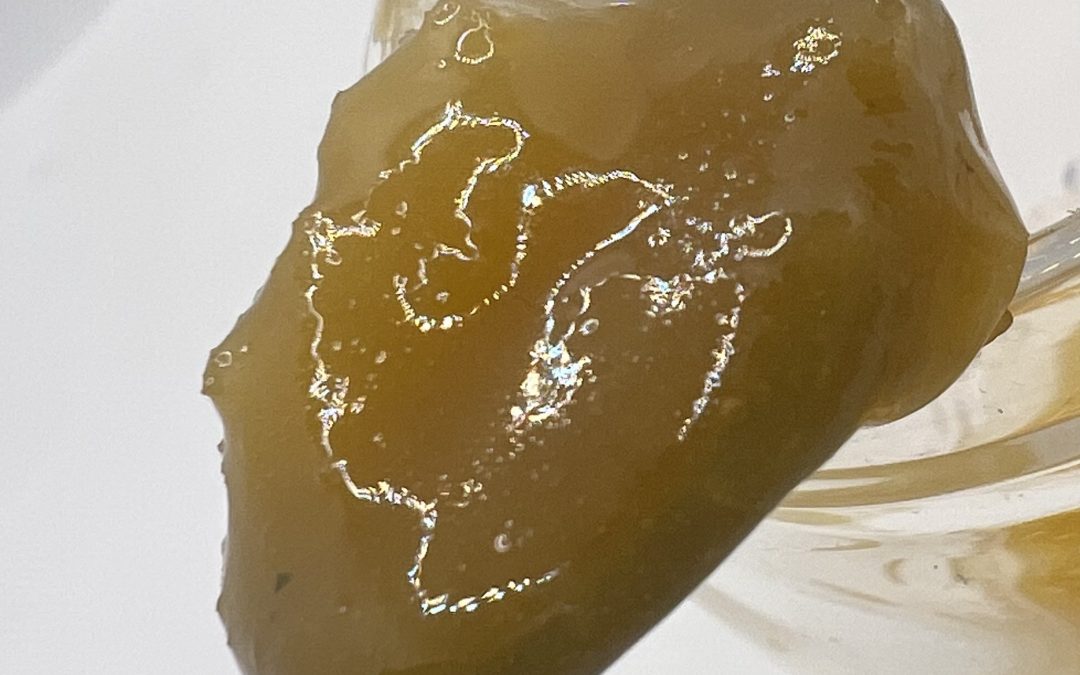
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid.
While we typically think of fluids as either a liquid or a gas, by manipulating pressure and temperature we can take a fluid (carbon dioxide, in our case) past its “critical” phase-change point to create a “supercritical” fluid — a unique state that exhibits the properties of both liquid and gas.
These properties make supercritical fluids extremely useful in extracting certain substances — flavors, active ingredients, essential oils — while leaving others behind. In its supercritical state, CO2 can diffuse through solids like a gas, and dissolve materials like a liquid. By precisely controlling variables like temperature, pressure and flow rate we can fine-tune the extraction process, customizing it to best suit the compounds we’re extracting
About the grower
SCFN CO2 extraction lab
Strain
Lemon haze soy latte
Breeder
Genetics
Hightech
Collaboration
Team Hightech
Mold
Nutrients
Medium
pest